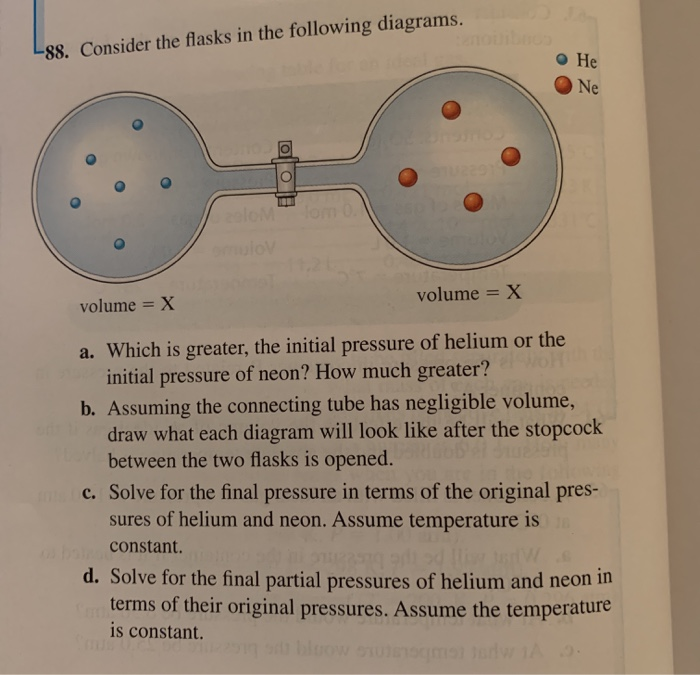
Flask X Contains 5Dm3 Of Helium . 4 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. The final volume will be 15 l. 5 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. 5 flask q contains 5 dm of helium at 12 kpa pressure. Flask r contains 10 dm of neon at 6 kpa pressure. Flask x contains 5 dm³, which is equal to 5 liters. 7 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa. q8 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa pressure. So the (partial) pressure of the helium decreases to 12*5/15 = 4. first, let's convert the volumes from dm³ to liters:
from www.chegg.com
first, let's convert the volumes from dm³ to liters: 5 flask q contains 5 dm of helium at 12 kpa pressure. So the (partial) pressure of the helium decreases to 12*5/15 = 4. 7 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa. 4 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. q8 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa pressure. Flask r contains 10 dm of neon at 6 kpa pressure. 5 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. Flask x contains 5 dm³, which is equal to 5 liters. The final volume will be 15 l.
Solved 88. Consider the flasks in the following diagrams. He
Flask X Contains 5Dm3 Of Helium So the (partial) pressure of the helium decreases to 12*5/15 = 4. 4 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. 5 flask q contains 5 dm of helium at 12 kpa pressure. first, let's convert the volumes from dm³ to liters: So the (partial) pressure of the helium decreases to 12*5/15 = 4. The final volume will be 15 l. Flask r contains 10 dm of neon at 6 kpa pressure. 7 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa. q8 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa pressure. Flask x contains 5 dm³, which is equal to 5 liters. 5 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure.
From solvedlib.com
A 255mL flask contains pure helium at a pressure of7… SolvedLib Flask X Contains 5Dm3 Of Helium first, let's convert the volumes from dm³ to liters: So the (partial) pressure of the helium decreases to 12*5/15 = 4. 5 flask q contains 5 dm of helium at 12 kpa pressure. Flask x contains 5 dm³, which is equal to 5 liters. The final volume will be 15 l. 4 flask x contains 5 dm. Flask X Contains 5Dm3 Of Helium.
From www.numerade.com
SOLVEDA 900.0mL flask contains 1.16 mg O2 and 0.42 mg He at 15^∘ C Flask X Contains 5Dm3 Of Helium q8 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa pressure. 4 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. Flask x contains 5 dm³, which is equal. Flask X Contains 5Dm3 Of Helium.
From byjus.com
11. Two flasks X and Y of volumes 250 ml and 300 ml respectively at the Flask X Contains 5Dm3 Of Helium The final volume will be 15 l. So the (partial) pressure of the helium decreases to 12*5/15 = 4. 5 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. q8 flask x contains 5 dm3 of helium at 12 kpa pressure and. Flask X Contains 5Dm3 Of Helium.
From byjus.com
11. Two flasks X and Y of volumes 250 ml and 300 ml respectively at the Flask X Contains 5Dm3 Of Helium 4 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. So the (partial) pressure of the helium decreases to 12*5/15 = 4. first, let's convert the volumes from dm³ to liters: 7 flask x contains 5 dm3 of helium at 12. Flask X Contains 5Dm3 Of Helium.
From askfilo.com
A two litre flask contains 22 g of carbon dioxide and 1 g of helium at 20.. Flask X Contains 5Dm3 Of Helium first, let's convert the volumes from dm³ to liters: So the (partial) pressure of the helium decreases to 12*5/15 = 4. 4 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. Flask r contains 10 dm of neon at 6 kpa pressure.. Flask X Contains 5Dm3 Of Helium.
From www.coursehero.com
[Solved] Consider the flasks in the following diagram. O O 2.50 L H2 1. Flask X Contains 5Dm3 Of Helium 4 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. first, let's convert the volumes from dm³ to liters: 7 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6. Flask X Contains 5Dm3 Of Helium.
From www.chegg.com
Solved 88. Consider the flasks in the following diagrams. He Flask X Contains 5Dm3 Of Helium So the (partial) pressure of the helium decreases to 12*5/15 = 4. q8 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa pressure. 5 flask q contains 5 dm of helium at 12 kpa pressure. 4 flask x contains 5 dm 3 of helium. Flask X Contains 5Dm3 Of Helium.
From material-properties.org
Helium Periodic Table and Atomic Properties Flask X Contains 5Dm3 Of Helium So the (partial) pressure of the helium decreases to 12*5/15 = 4. Flask r contains 10 dm of neon at 6 kpa pressure. 5 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. q8 flask x contains 5 dm3 of helium at. Flask X Contains 5Dm3 Of Helium.
From www.numerade.com
SOLVED As shown in Fig. 162, two flasks are connected by an initially Flask X Contains 5Dm3 Of Helium 5 flask q contains 5 dm of helium at 12 kpa pressure. Flask x contains 5 dm³, which is equal to 5 liters. first, let's convert the volumes from dm³ to liters: Flask r contains 10 dm of neon at 6 kpa pressure. 5 flask x contains 5 dm 3 of helium at 12 kpa pressure and. Flask X Contains 5Dm3 Of Helium.
From www.chegg.com
Solved A 12.5L scuba diving tank contains a heliumoxygen Flask X Contains 5Dm3 Of Helium q8 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa pressure. Flask r contains 10 dm of neon at 6 kpa pressure. 5 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at. Flask X Contains 5Dm3 Of Helium.
From www.numerade.com
SOLVED Consider two flasks connected by a stopcock. One flask has a Flask X Contains 5Dm3 Of Helium So the (partial) pressure of the helium decreases to 12*5/15 = 4. 7 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa. 4 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at. Flask X Contains 5Dm3 Of Helium.
From www.numerade.com
Each of four flasks is filled with a different gas. Each flask has the Flask X Contains 5Dm3 Of Helium Flask x contains 5 dm³, which is equal to 5 liters. Flask r contains 10 dm of neon at 6 kpa pressure. first, let's convert the volumes from dm³ to liters: 7 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa. 4 flask x. Flask X Contains 5Dm3 Of Helium.
From askfilo.com
temperature is different Q.10 A 0.5dm3 flask contains gas 'A' and 1dm3.. Flask X Contains 5Dm3 Of Helium So the (partial) pressure of the helium decreases to 12*5/15 = 4. 5 flask q contains 5 dm of helium at 12 kpa pressure. 7 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa. 5 flask x contains 5 dm 3 of helium at. Flask X Contains 5Dm3 Of Helium.
From www.toppr.com
A cylinder contains a mixture of helium and argon gasin equilibrium at Flask X Contains 5Dm3 Of Helium 4 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. 5 flask q contains 5 dm of helium at 12 kpa pressure. Flask r contains 10 dm of neon at 6 kpa pressure. Flask x contains 5 dm³, which is equal to. Flask X Contains 5Dm3 Of Helium.
From www.numerade.com
SOLVED10.^ 1,295 ml flask contains pure helium at pressure of 74SmmHg Flask X Contains 5Dm3 Of Helium 7 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa. Flask r contains 10 dm of neon at 6 kpa pressure. The final volume will be 15 l. first, let's convert the volumes from dm³ to liters: 5 flask q contains 5 dm of. Flask X Contains 5Dm3 Of Helium.
From www.numerade.com
SOLVEDEach box in Figure 11.11 represents the contents of a flask. One Flask X Contains 5Dm3 Of Helium 5 flask q contains 5 dm of helium at 12 kpa pressure. So the (partial) pressure of the helium decreases to 12*5/15 = 4. 5 flask x contains 5 dm 3 of helium at 12 kpa pressure and flask y contains 10 dm 3 of neon at 6 kpa pressure. first, let's convert the volumes from dm³. Flask X Contains 5Dm3 Of Helium.
From www.numerade.com
SOLVED O2 Valve 1 He Valve 2 Valve 3 N2 5. Three 1liter flasks are Flask X Contains 5Dm3 Of Helium q8 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa pressure. first, let's convert the volumes from dm³ to liters: 5 flask q contains 5 dm of helium at 12 kpa pressure. The final volume will be 15 l. So the (partial) pressure of. Flask X Contains 5Dm3 Of Helium.
From byjus.com
Two flask x y have capacity 1L 2L respectively each of them contains 1 Flask X Contains 5Dm3 Of Helium So the (partial) pressure of the helium decreases to 12*5/15 = 4. q8 flask x contains 5 dm3 of helium at 12 kpa pressure and flask y contains 10 dm3 of neon at 6 kpa pressure. 5 flask q contains 5 dm of helium at 12 kpa pressure. first, let's convert the volumes from dm³ to liters:. Flask X Contains 5Dm3 Of Helium.